Leukippos and Demokritos of the fifth century BC conceived of matter as consisting of myriads of minute, indivisible particles called atoms. The variety and behaviour of matter was explained in terms of the arrangement and motions of these atoms. The concept was elaborated in the poem De Rerum Natura (On the nature of things) by the first-century BC Roman poet Lucretius, but fell into obscurity, from which it was not rescued until the revival of atomism in the seventeenth century. From 1803, John Dalton developed his atomic theory, that is, that the atoms of each chemical element were identical but different from those of other elements in one respect: they had different weights (now called relative atomic masses).
He also indicated the ways in which compound atoms, or molecules, could be formed by combinations of atoms and embarked on the determination of the weights of the atoms of various elements, that is, the number of times heavier they were than the lightest known element, hydrogen. Progress was promising at first, but some anomalous results cast doubt on the possibility of working out atomic weights and led to half a century of confusion, when it was said in despair that each chemist had his own system of atomic weights. At the Karlsruhe Conference of 1860, sound rules were finally established and from then on, reliable and accurate values for atomic weights became available to chemists.
In 1869, Dimitri Mendeleev in Russia and Lothar Meyer in Germany arranged the elements in ascending order of atomic weight, noting that elements with similar properties formed themselves into classes or group. This was a step towards understanding the relationship between the elements, which hitherto seemed to be unrelated, isolated individuals. But the atoms were still regarded, until the end of the century, as solid, indivisible, indestructible particles of matter, obedient to the traditional Newtonian laws of motion.
A series of discoveries around the turn of the century shattered this image. By 1897, J.J.Thomson had established the existence of the electron, a particle with a negative electric charge only a minute part of the weight of an atom. From 1895, Henri Becquerel and Marie and Pierre Curie explored the radioactivity of the heavy elements such as uranium and radium, which were disintegrating spontaneously with the release of energy and minute particles of matter. Albert Einstein showed that matter and energy are interchangeable, a very little mass being equivalent to a very large amount of energy, related to each other by the now celebrated equation E=mc2 , where E is the energy, m the mass and c the velocity of light. After the work of Frederick Soddy and Ernest Rutherford during the first decade of this century, Niels Bohr was able to propose in 1913 a model for the atom entirely different from the traditional view.
The mass of the atom was concentrated in a positively charged nucleus at the centre and sufficient negatively charged electrons circling round it to leave the atom electrically neutral. During the 1920s a new system of mechanics, quantum mechanics, was developed as, at the atomic level, traditional mechanics no longer applied. The neutron, a particle identical in weight to the proton but having no electrical charge, was discovered by Chadwick in 1932. From then on, the atom was visualized as a nucleus made up of protons and neutrons with, circling round it in orbits, a number of electrons equal to the number of protons. The latter is the atomic number and determines the chemical identity of the element. The number of protons and neutrons is the mass number.
Atoms of the same element with the same atomic number can have a slightly different number of neutrons: these are known as isotopes. Thus, most uranium (atomic number 92) has in its nucleus 146 neutrons, giving a mass number of 238. But there is another isotope of uranium with only 143 neutrons, known as uranium (U-235). Around the mid-thirties, scientists were trying to obtain artificial elements, heavier than uranium, with nuclei so unstable that they could not occur in nature. The most promising method was to bombard uranium atoms with slow neutrons in a particle accelerator. Hahn and Strassman were adopting this method in 1938 and expected to detect the element with atomic number 93 but instead found indications of an element similar to barium, with an atomic number about half that of uranium.
This was puzzling and it was the Austrian physicists Lise Meitner and Otto Frisch who published the correct explanation in two famous letters in Nature in February 1939: the neutrons had split each uranium atom into two medium-sized ones with the liberation of further neutrons and a considerable amount of energy. Later that year, Bohr and Wheeler suggested that the liberated neutrons could split more atoms, with production of yet more neutrons, and so on—a chain reaction. Two days after this publication appeared, the Second World War broke out.


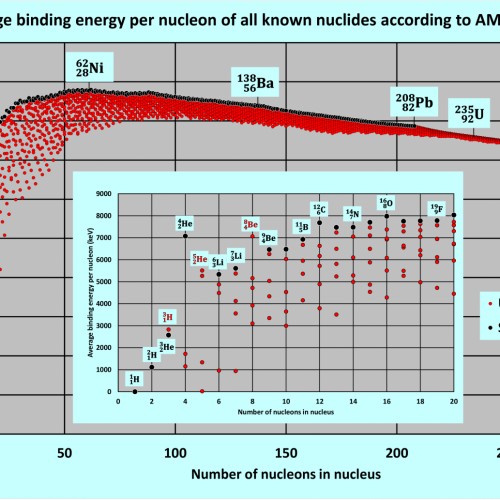 Nuclear Binding Energy
Nuclear Binding Energy














































